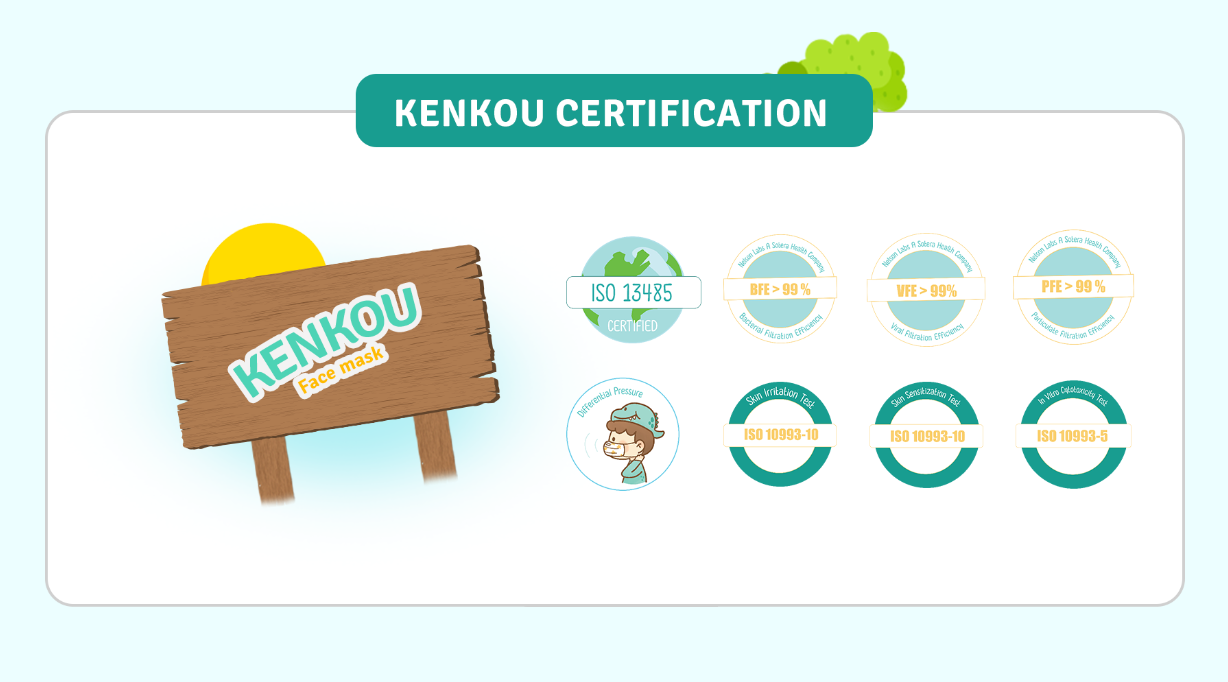

ISO 13485
A system of quality management standards specific to the medical device industry that covers the design, development, manufacture and sale of medical devices as an international system.

BFE > 99%
Bacterial Filtration Efficiency (BFE) is a value that indicates the ability of a mask to filter particles of bacteria and other microorganisms with a mean particle size (MPS) of 3 ± 0.3 microns.
KENKOU FACE MASK: BFE = 99.9% (ASTM Level 3) indicates a high bacterial filtration efficiency.
KENKOU FACE MASK: BFE = 99.9% (ASTM Level 3) indicates a high bacterial filtration efficiency.

VFE > 99%
Viral Filtration Efficiency (VFE) is a value that indicates the ability of a mask to filter viral particles with a mean particle size (MPS) of 3 ± 0.3 microns.
KENKOU FACE MASK : VFE = 99.9%
KENKOU FACE MASK : VFE = 99.9%

PFE > 99%
Particulate Filtration Efficiency (PFE) is a value that indicates the ability of a mask to filter very small particles with particles size of 0.1 microns, so it can filter PM2.5 as well.
KENKOU FACE MASK: PFE = 99.7% (ASTM Level 3) indicates a high level of protection against ultrafine particles.
KENKOU FACE MASK: PFE = 99.7% (ASTM Level 3) indicates a high level of protection against ultrafine particles.

Delta P (Differential Pressure)
It is a measure of the air resistance of the mask to see the ease of breathing. The lower the resistance, the easier it is to breathe. As a standard, the value must be less than 5 mm H2O/cm2.
KENKOU FACE MASK: Delta P = 3.8 mm H2O/cm2 (ASTM Level 3) indicates comfortable breathing but still high filtration efficiency.
KENKOU FACE MASK: Delta P = 3.8 mm H2O/cm2 (ASTM Level 3) indicates comfortable breathing but still high filtration efficiency.

Flammability
Since hospitals have many sources of ignition such as oxygen, heat, and chemicals, flammability testing is necessary. Medical masks are required to withstand fire when exposed to flames for a period of more than or equal to 3.5 seconds.
KENKOU FACE MASK: Class 1 Flammability, Burn Code Description = IBE indicates the ability to withstand flammability for a specified period of time and can be extinguished.
KENKOU FACE MASK: Class 1 Flammability, Burn Code Description = IBE indicates the ability to withstand flammability for a specified period of time and can be extinguished.

Resistance to Penetration
by Synthetic Blood
The ability to prevent penetration of the liquid (Fluid Resistance) from the outer layer to the inner layer of the mask using artificial blood to test the penetration at a pressure of 80 mmHg (Low Barrier), 120 mmHg (Medium Barrier), and 160 mmHg (High Barrier).
KENKOU FACE MASK: passed at 160 mmHg (ASTM Level 3) indicates a high level of protection against liquid penetration.
KENKOU FACE MASK: passed at 160 mmHg (ASTM Level 3) indicates a high level of protection against liquid penetration.

In Vitro Cytotoxicity Test
ISO 10993-5
Products passed In Vitro Cytotoxicity Test
ISO 10993-5
Products passed In Vitro Cytotoxicity Test
·

·
Skin Irritation Test
ISO 10993-10
ISO 10993-10
Products passed the test and confirmed to be free of skin irritants.

Skin Sensitization Test
ISO 10993-10
ISO 10993-10
Products passed the test and confirmed to be free from substances that cause skin hypersensitivity.